filter integrity test machine|filter integrity test steps : distributing The introduction of FDA guidance and European Good Manufacturing Practice (GMP) (regulators) in 1987 of mandatory integrity testing of critical use filters, accelerated the need for high performance automated test equipment. By the mid-to-late 90s recommendations for filter integrity testing by the regulatory bodies had expanded. Acompanhe as últimas notícias, resultados, escalações e opiniões sobre o Tricolor Paulista. Saiba tudo sobre o clássico contra o Santos, a situação de James Rodríguez e .
{plog:ftitle_list}
ARCHIE MADEKWE nude scenes - 2 images and 2 videos - including appearances from "Saltburn".
The Sartocheck® 5 Plus Filter Integrity Tester sets new standards for filter integrity testing devices and combines a unique approach to meet today's industry requirements.
Perform the integrity test in a lower surface tension reference fluid to assess filter wettability changes independent of filter integrity. If the filter fails using the reference fluid, the filter fails .Integrity testing sterilizing filters is a fundamental requirement of critical process filtration applications in the pharmaceutical industry. - Find MSDS or SDS, a COA, data sheets and more information.
The introduction of FDA guidance and European Good Manufacturing Practice (GMP) (regulators) in 1987 of mandatory integrity testing of critical use filters, accelerated the need for high performance automated test equipment. By the mid-to-late 90s recommendations for filter integrity testing by the regulatory bodies had expanded.Application Note: Integrity Test Troubleshooting - Beyond Rewet and Retest. Integrity testing is a critical operation, especially for sterilizing grade filters used in biopharmaceutical processing. When performed correctly, an integrity test is a fast, definitive, non-destructive way to assure filter retention performance.
Filter integrity testing is a critical step in manufacturing of sterile drug products. With regulatory agencies and the Parenteral Drug Association (PDA) recommending pre-use and requiring post-use integrity testing, you can rely on our integrity testing to follow industry best practices — not only establishing a product-specific filter integrity test specification, but rigorously checking .Establish a Product-Specific Filter Integrity Test Specification Filter integrity testing is a critical step in manufacturing sterile drug products. Regulatory agencies and the Parenteral Drug Association (PDA) recommend pre-use and require post-use integrity testing to check for leaks or filter damage in the sterilizing-grade filters used to .Automated integrity test instrument. Consistent with Meissner’s commitment to developing smart products that bolster quality, reduce risk and provide a second to none customer experience, the AccuFlux ® filter integrity testing machine is engineered to combine the highest levels of instrument accuracy, robustness, and ease of use. In an era where importance of quality and .Biopharmaceutical Manufacturing > Downstream Processing > Sterile Filtration > Integrity Testing C189432Integrity assurance with ease and confidence The Integritest ® 5 is an easy-to-use, portable, and fully-automated instrument that verifies the .
Filter integrity testing, from its origins in the 1970s, has undergone significant changes over the last 40 years as industry needs, and the evolution of regulatory expectations have . test equipment. By the mid-to-late 90s recommendations for filter integrity testing by the regulatory bodies had expanded. US regulators recommended integrity .
HEPA filter integrity testing of negative air units, vacuums and any other HEPA filtered equipment on abatement and remediation projects. This guideline is considered a . training in the use of the testing equipment and the required procedures for field testing noted in this guideline. In-house training should be provided by a competent .The BP integrity test using the Flowstar IV integrity test instrument features this stabilization/leak test before proceeding to the actual stepwise increase of pressure for the BP test. This initial leak test seeks to establish that the filter system under test shows an expected and typical background flow. Integrity testing of filters is crucial to confirm the reliability of filtration processes and follow the regulatory compliance stated by GMP standards and the FDA. Once filtration goals are established and the system is operational, integrity testing is the most certain way for system operators to know if the filters are working as required .
The dispersed oil particulate (DOP) scan testing, also known as filter integrity testing, or leak testing, is one of the most quoted methods by industry standards. . 2i Aerosol Photometer The global expert in the design and manufacture of specialised testing equipment for HEPA filters and protective masks has released its new product; iProbe .
Bubble point tests may be used on single round cartridge or crossflow filters. A bubble point test is a non-destructive method of integrity testing that allows the user to correlate their results with manufacturer-determined values that indicate proper function. Filter integrity testing plays a pivotal role in assessing the reliability and efficiency of membrane filters, especially in critical applications such as sterile filtration. . By using these methods and associated test equipment, users can ensure the reliability and optimal functioning of membrane filters in varied applications. 2 3 .Clean, dry air or nitrogen source of 1.4 – 8.2 bar (20 – 120 psi) at least 1 bar (15 psi) greater than the highest test pressure: Operating Temperature Range: 1 to 40 °C: Type of Test: Bubble Point; Diffusion; HydroCorr; Water-based integrity test HydroCorr; Quality Level: EQ3Integrity Testing Fit for Use Focus on These 3 Elements of Validation Sterile Filter Master Extractables/ Plan Prove the stream does not adversely impact the filter. Identify, quantify, and assess impact of compounds that migrate from filter to process stream. Prove the filter removes bacteria from the stream per ASTM 838-05.
With the known sensitivity limitations of compendial sterility tests, filter integrity is a critical control point contributing to the confirmation and release of a drug product as sterile. The good news is that with modern automated equipment filter integrity testing has become fast, accurate, reproducible, traceable, and auditable.monitors filter integrity test activity across multiple Palltronic Flowstar V instruments. It can improve productivity by providing insights into: • Test results by instrument, filter product and individual operators • Troubleshooting false-failure integrity test results • Preventive maintenance and calibration scheduling Fig 3.The Donaldson “Membra-Check” is a handy filter integrity test unit for both hydrophilic and hydrophobic membrane filters. In addition to all integrity tests, this unit can be used as a calibration pressure gauge (0 – 6 bar) and is capable of checking unknown volumes (e.g. the upstream volume of a filter housing; 0.1l – 32l).
canvas test question with a multiple answer drop down
Biopharmaceutical Manufacturing > Downstream Processing > Sterile Filtration > Integrity Testing C189432Integrity assurance with ease and confidence The Integritest ® 5 is an easy-to-use, portable, and fully-automated instrument that verifies the .Establish a Product-Specific Filter Integrity Test Specification Filter integrity testing is a critical step in manufacturing sterile drug products. Regulatory agencies and the Parenteral Drug Association (PDA) recommend pre-use and require post-use integrity testing to check for leaks or filter damage in the sterilizing-grade filters used to .The 100X Automated Filter Tester is designed for test and quality control validation of filter media, replaceable particulate filters, and masks used in medical and industrial hygiene applications. The 100X meets leading global industry standards such as NIOSH 42 CFR Part 84, GB 2626, EN 13274-7:2019, ASTM F3502-21, and ASTM F2100-23. The test machine could report such a large pressure loss as a gross leak failure. . Multi-Round Virus Filter Integrity Test Sensitivity. Biotechnol. Bioeng 103:574-581. 5.) Hofmann, R. 1984. Integrity Testing of Microfiltration Membranes. PDA J 38:148-159. 6.) P17512 Rev 1998.
Also, filter integrity testing often involves flammable alcohol. For example, if an integrity test fails, the PDA recommends retesting (1, 7). Some nine out of 10 failures are attributable to improper wetting, so the third and final test should be performed afterFilter-integrity testing is an essential step for a batch release. A false-passed integrity test (e.g., a conforming test result even though a filter is broken) could risk the patient health if it is not detected . Filter Integrity Machine (Palltronic Flowstar IV), is 21 CFR compliance machine installed in Grade D of–Filter may have a hole in it that is plugged as part of the filtration process –May then pass a post-use integrity test, but batch is not sterile –Filter may become damaged by the sterilisation process –Filter may not be sitting in its housing or connected correctly –Integrity testing the filter before and after its use helps

The most-common onsite HEPA integrity test method is the Cold DOP test, as described in AS1807.4.4 and 1807.7. At the conclusion of our comprehensive testing, you will receive a detailed analysis report and a NATA accredited certificate of compliance to verify HEPA filter integrity as per Australian Standard specifications.
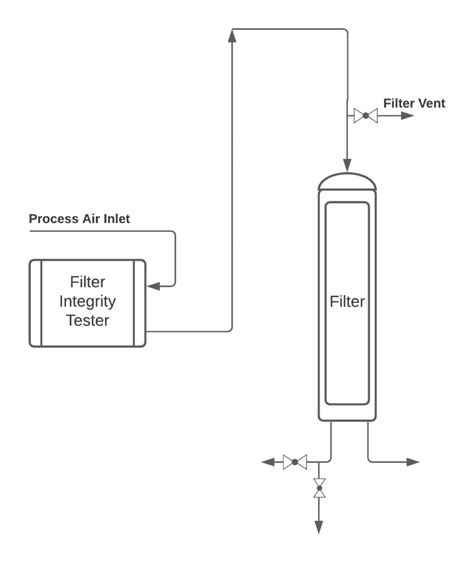
types of filter integrity test
If you’re having trouble, feel free to drop us a line. Please dire.
filter integrity test machine|filter integrity test steps